It’s been another interesting 12 months in ‘spine’ – proving the industry is alive and thriving – with highlights including:
Spin-offs Zimmer Biomet
Mergers Integrity Implants and Fusion Robotics becoming Accelus
Acquisitions SeaSpine acquiring 7D Surgical, Nuvasive acquiring Simplify Medical, ATEC acquiring EOS Imaging
Name changes HT Medical becoming Xenix Medical
Product innovation Nanovis now has the first FDA nano registered pedicle screw system
Investment MiRus closing $65m
Last year before the “Virtual NASS”, I compiled a list of six up-and-coming start-ups within the ‘spine’ arena including a short summary of each business. This year, I have compiled a similar list of ‘ones to watch’ for 2021/22. These seven companies might have an innovative product or a favourable market position for growth over the next 12 months.
Accelus – Expandable cages meet robotics
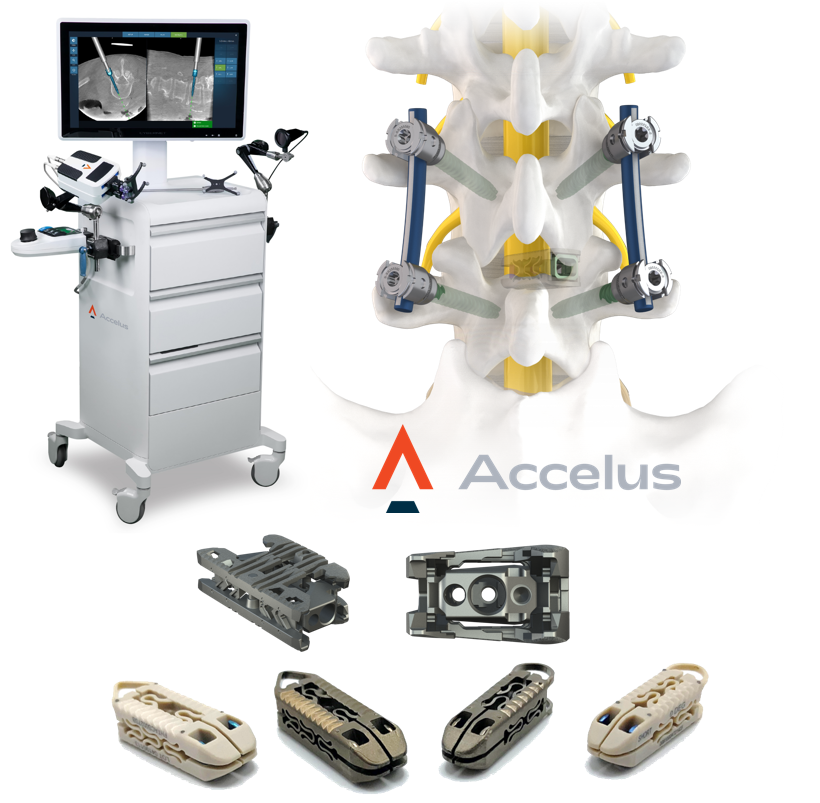
Accelus was founded in July 2021, through the combination of Integrity Implants and Fusion Robotics, and is focused on accelerating the move to minimally invasive spine surgery with its Adaptive Geometry™ technology, which fundamentally respects patients’ neural, vascular, bony, and soft tissue anatomy both during and after insertion to promote long-term stability. In addition, its Robotic Navigation System eliminates previous robotics barriers – like system cost, footprint, setup, tear-down, and workflow inefficiencies – to allow for the wide-scale adoption of robotics in spine surgery.
Accelus will launch its Robotic Navigation System, with the company’s LineSider® Spinal System, at NASS 2021. A recently published study* showed the system has a significantly shorter procedure workflow duration while maintaining the same accuracy as the most commonly used robotic platform in spine and at a fraction of that system’s cost. Accelus will also launch its latest evolution in Adaptive Geometry with the Toro™-L Biplanar Expandable Interbody Fusion System. Toro is redefining lateral with expansion mechanics that allow it to expand to its full width before seamlessly transitioning into its height cycle, 3D-printed endplates, , an additive manufacturing process that creates a surface roughness of 5-10 microns where the implant interfaces with the bone, and a large graft delivery window and open architecture to allow for in- and through-filling of bone graft. Accelus will also feature its flagship FlareHawk® and TiHawk™ family of expandable interbody cages. To date, more than 10,500 FlareHawk cages have been implanted in more than 9,000 patients.
*Soliman M A, Khan A, O’Connor T E, et al. (June 26, 2021) Accuracy and Efficiency of Fusion Robotics™ Versus Mazor-X™ in Single-Level Lumbar Pedicle Screw Placement. Cureus 13(6): e15939. doi:10.7759/cureus.15939
Carbo-Fix – Next generation of carbon implants for tumour care
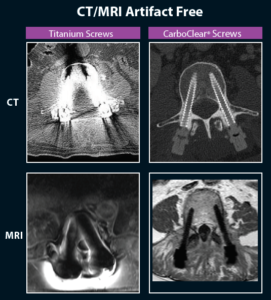
The CarboClear Pedicle screw system is designed and cleared specifically for the treatment of spine oncology patients. It is the only FULLY carbon fibre system approved for sale in the US.
Why CarboClear is the right choice for spine oncology patients:
- Carbon Fiber Reinforced PEEK implants produce virtually no artefacts under CT or MRI imaging.
- CFR-PEEK with an ultra-thin titanium coating to enhance bony integration and allow the surgeon to visualise the entire implant for trajectory and accurate placement.
- Improved pre-radiotherapy planning – this system eliminates the uncertainty caused by titanium implants.
- Treatment:
Reduced interference with radiotherapy protocols: no backscattering or attenuation
Protect surrounding tissue from overdose
Allows for proton therapy - Superior Fatigue Resistance over metal implants.
CarboClear will be introducing its new and improved full carbon fiber system at NASS Boston 2021. It is also planning to release more full carbon fibre options in 2021.
This is in addition to its currently approved spine oncology pedicle system and its degenerative products, VBR, lumbar cage and cervical plate.
DiFusion Technologies – New clean, bioactive polymer
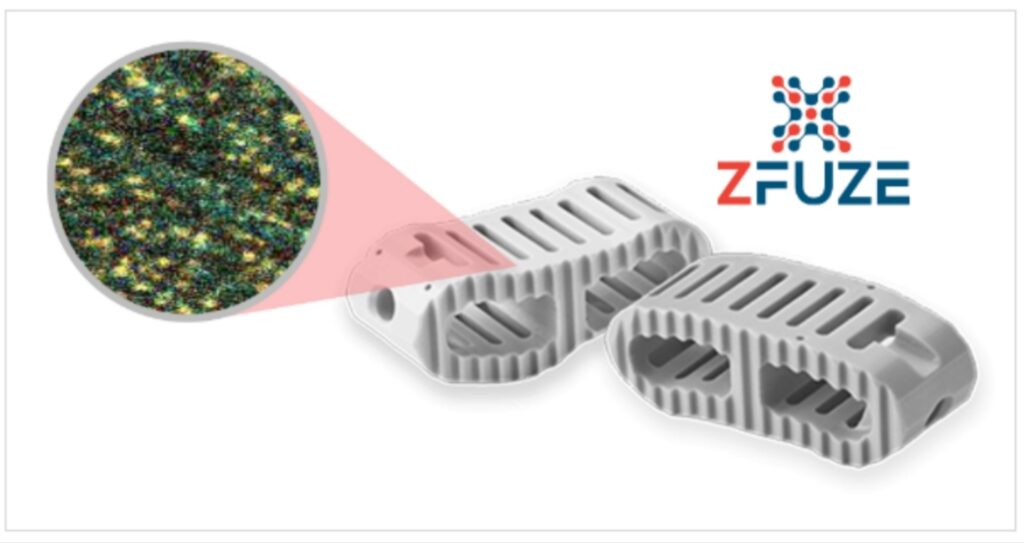
By definition, biomaterials are foreign bodies. Historically, their design has largely been based upon mechanical and physical properties, and bioinertness was a desirable characteristic. Today, as the adaptability of immune cells and the role that biomaterials play on immune-mediated tissue responses becomes better understood, there is a shift towards designing materials that can proactively modulate the immune system. ZFUZE is the first biomaterial with a complete Biocompatibility FDA Master File that was engineered to avoid the Human Foreign Body Response which results in fibrous encapsulation of implants historically used in Spinal Fusion procedures by large OEM’s . DiFusion has begun a large Patient Outcome Study at the Rothman Institute (Philadelphia) which will be the definitive biomaterial study for efficacy in Spinal Fusion to be completed in early 2022.
Located in Austin Texas, DiFusion was founded in 2009 and has been dedicated to engineering and bringing to market a suite of patented Immunomodulatory, Tissue Regenerating and Antimicrobial Polymers in the Spinal, Sports Medicine and Total Joint markets. DiFusion has assembled a team of leading clinical, regenerative medicine and research experts on their Scientific Advisory Board.
DuraStat – A breakthrough device for dural repair
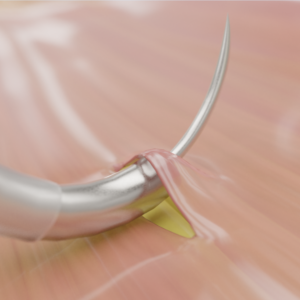
Incidental durotomies, or dural tears, are one of the most common complications in spine surgery, yet the tools available to surgeons for efficient, high-quality intraoperative treatment have historically been limited. DuraStat addresses the key limitations of conventional repair tools and techniques to help surgeons create high-quality dural closures with safety, reproducibility, and efficiency, even in the most difficult situations. The curved blunt tip with recessed needle and suture allows surgeons to select the ideal position safely and precisely for their repair. The needle and suture are deployed with a button-press, allowing single-handed needle passage by incorporating standard suture technique (supination) into the device.
Over the next 12 months, DuraStat plans to transition from being regionally accessible to nationally available, while also developing ways to apply the novel design to solve other prominent unmet clinical needs. The recent increased interest in converting to minimally invasive procedures and migrating to outpatient surgical environments has magnified the importance of innovating in the area of intraoperative complication management. DuraStat aims to partner with surgeons as they make these procedural and site-of-care migrations, while contributing to net-cost-savings for facilities and better outcomes for patients.
Elevation Spine – Traditional fusion redefined
Elevation Spine is a medical device company with a proprietary technology platform – Saber Technology – which is an advanced and differentiated approach to traditional fusion.

Saber Technology was designed to improve patient outcomes while providing surgeons with a high-tech, yet simple approach to spinal fusion procedures. The first FDA-cleared Saber Technology product, SABER-C™, is comprised of a zero-profile anterior cervical plate that accepts multiple spacer material and fixation options and provides a proprietary single-step integrated-fixation. Traditional fusion technologies utilise a plate and screws to fixate the spine during a fusion procedure. In contrast, SABER-C’s robust and versatile implant offering utilises a unique curved spike device which eliminates the large working space that a straight screw requires, therefore minimising incision size and simplifying the surgical technique. As a result, the Elevation Spine fusion procedure is extremely efficient while remaining minimally invasive.
Over the next few months, Elevation Spine is laser focused on scaling its technology via a capital raise of its series B and hiring sales management.
Next Orthosurgical – Multi-billion dollar backed business looking to grow
Next Orthosurgical is a Vista, CA medical device developer, manufacturer and distributor. It is a subsidiary of a multi-billion-dollar Japanese medical device company with plans for rapid growth in the United States, which will be accomplished through development of existing 510(k) approved products combined with a sound acquisition and licensing strategy. The company recently launched a corrective angle technology (CAT) MIS pedicle screw to accommodate anatomic MIS tab challenges in the lumbar spine. Next Orthosurgical acquired VTI through an asset sale in February 2020 and will seek FDA approval to re-launch the Interfuse technology in a 3D titanium version in early 2022. It recently expanded manufacturing capacity to accommodate anticipated growth and has plans to build a state-of-the-art bioskills lab and training center adjacent to the corporate headquarters in Vista, CA. It is in the process of revamping the company website, messaging and its social media presence.
Next Orthosurgical is a surgeon-centric start-up that recognises success through new product development would not be possible without the collaboration of top spine surgeons in the US. It has plans to add several Regional Vice Presidents, to expand growth within the independent distributor network in the US. The company is also hiring for engineering, marketing, operations and customer service positions in Vista, CA.
TDi (Tissue Differentiation Intelligence) – AI-driven intraoperative soft-tissue imaging

TDi is deploying Artificial Intelligence (AI) to create a first-of-its-kind ultrasound imaging technology specifically designed for spine surgery. SonoVision, the company’s flagship technology platform, applies Machine Learning (ML) to real-time ultrasonic images, delivering a simple, intuitive and procedurally relevant view of soft-tissue structures to operative surgeons.
The initial SonoVision application is focused on streamlining nerve identification during lateral access surgery, which the company plans to progress rapidly towards commercialisation in the coming quarters. Additional pipeline applications for the technology – particularly related to transforaminal and endoscopic access – will also be a near-term focus.
If you have any thoughts on the state of the spine market, I’m always interested to learn about the next big thing, so please don’t hesitate to reach out.
Matthew Henshaw is the Founder and CEO of Talanoa, he acts as a recruitment strategist and startup mentor in the medical device industry.
Connect with Matthew on LinkedIn and feel free to talk about your business and recruitment struggles. Let’s see if I can help with my expertise or my own network.



